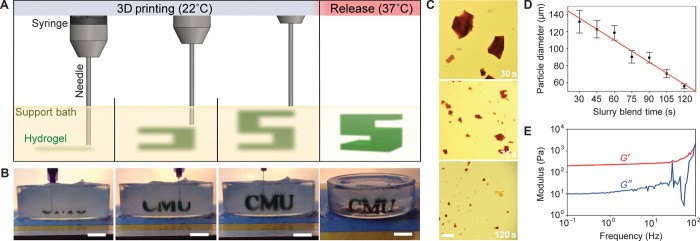
Header Image: Scheme (A) and image (B) of the FRESH process showing the gel of interest (A. green, B. black) printed in the temporary support gel. After printing layer by layer, the 3D structure is released by melting the support gel.
A critical size bone defect is defined as the absence of a bone segment that would not heal spontaneously and require surgical intervention to make the bone functional again. The exact size and volume of a critical size bone defect depend on the patient and the location, but in most cases refers to a defect that is greater than 1 cm in length and greater than 50% of the circumference of the bone in volume. Critical size bone defects are the result of pathologies or traumatisms such as infections, tumor resections, non-union fractures or accidents and lead to a difficult clinical scenario for the surgeon and the patient. As the bone is not able to insure bone regeneration to fill the gap, several surgical procedures promoting osteogenesis (bone formation) are currently used like the Induced Membrane Technique, Distraction Osteogenesis and autologous (from the patient) grafting. These procedures present several limitations such as multiple surgical interventions, infection, long duration of reconstruction, donor site morbidity. There is an un-meet clinical need for scaffolds supporting bone formation and viable osteointegration in a cost-effective manner while minimizing patient morbidity (complications). 3D printed implants represent a promising alternative currently under investigation in the orthopaedic research field [1].
Within the ETN BioTrib working on orthopaedic implant technology, some projects aim at making progress towards the development of biodegradable scaffolds specific to patients’ bone defects. 3D bioprinting according to a design following the complex internal architecture of bone with collagen (main bone component) and autologous cells would combine interesting features to support bone regeneration while the implant is degraded. However, reaching mechanically stable scaffolds (not collapsing or deforming under their own weight) over several millimeters with good resolution can be hard when printing superimposed layer of soft gels. The emerging FRESH (Freeform Reversible Embedding of Suspended Hydrogels) bioprinting technology, first described in 2015 by Dr. Feinberg’s group, represents a promising approach to overcome this limitation, as collagen is printed into a secondary gel used as a temporary support.
The main advantage is to maintain the intended 3D geometry using biologically relevant soft gels that would collapse if printed in air. Once the 3D structure is FRESH printed, the support gel is discarded through melting while maintaining the stability and resolution of the collagen 3D structure. FRESH scaffolds present limitations encountered in 3D bioprinted scaffolds, namely vascularization of thick scaffolds, and ability to resist loading which is needed for bone implants. FRESH bioprinting of functional implant for critical size bone defects requires further development, but this technology expands the bioprinting possibilities in academia and industry [2].
References
[1] Mayfield, C. K., Ayad, M., Lechtholz-Zey, E., Chen, Y., & Lieberman, J. R. (2022). 3D-Printing for Critical Sized Bone Defects: Current Concepts and Future Directions. Bioengineering, 9(11), 680. https://doi.org/10.3390/bioengineering9110680
[2] Hinton, T. J., Jallerat, Q., Palchesko, R. N., Park, J. H., Grodzicki, M. S., Shue, H. J., Ramadan, M. H., Hudson, A. R., & Feinberg, A. W. (2015). Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Science Advances, 1(9). https://doi.org/10.1126/sciadv.1500758

This article was written by Marie Moulin as part of an ongoing series of scientific communications written and curated by BioTrib’s Early Stage Researchers.
Marie is researching the Bioprinting of Bone and Cartilage at Uppsala University, Sweden.