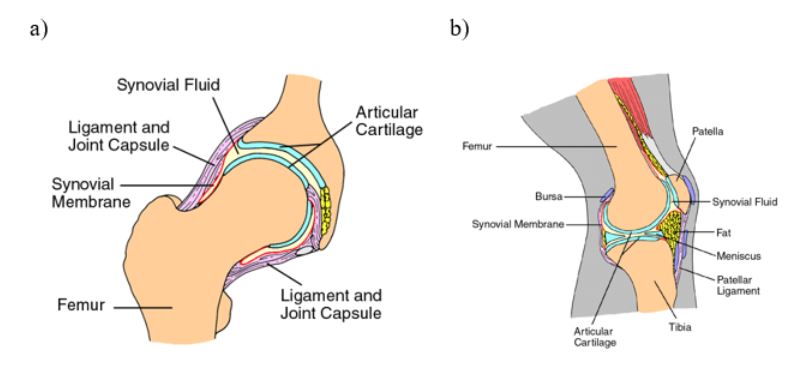
3D printing is rapidly advancing, with a significant expansion in the range of available materials. Previously confined to fast-curing polymers, the technology can now accommodate slow-curing polymers, offering distinct advantages such as enhanced elastic properties, durability, and robustness.
This breakthrough is attributed to a novel technology developed by researchers at ETH Zurich in collaboration with a US start-up. Consequently, researchers can now employ a variety of high-quality materials to 3D print intricate and more durable robots in a single process. The technology also facilitates the seamless combination of soft, elastic, and rigid materials, enabling the creation of delicate structures and parts with desired cavities.
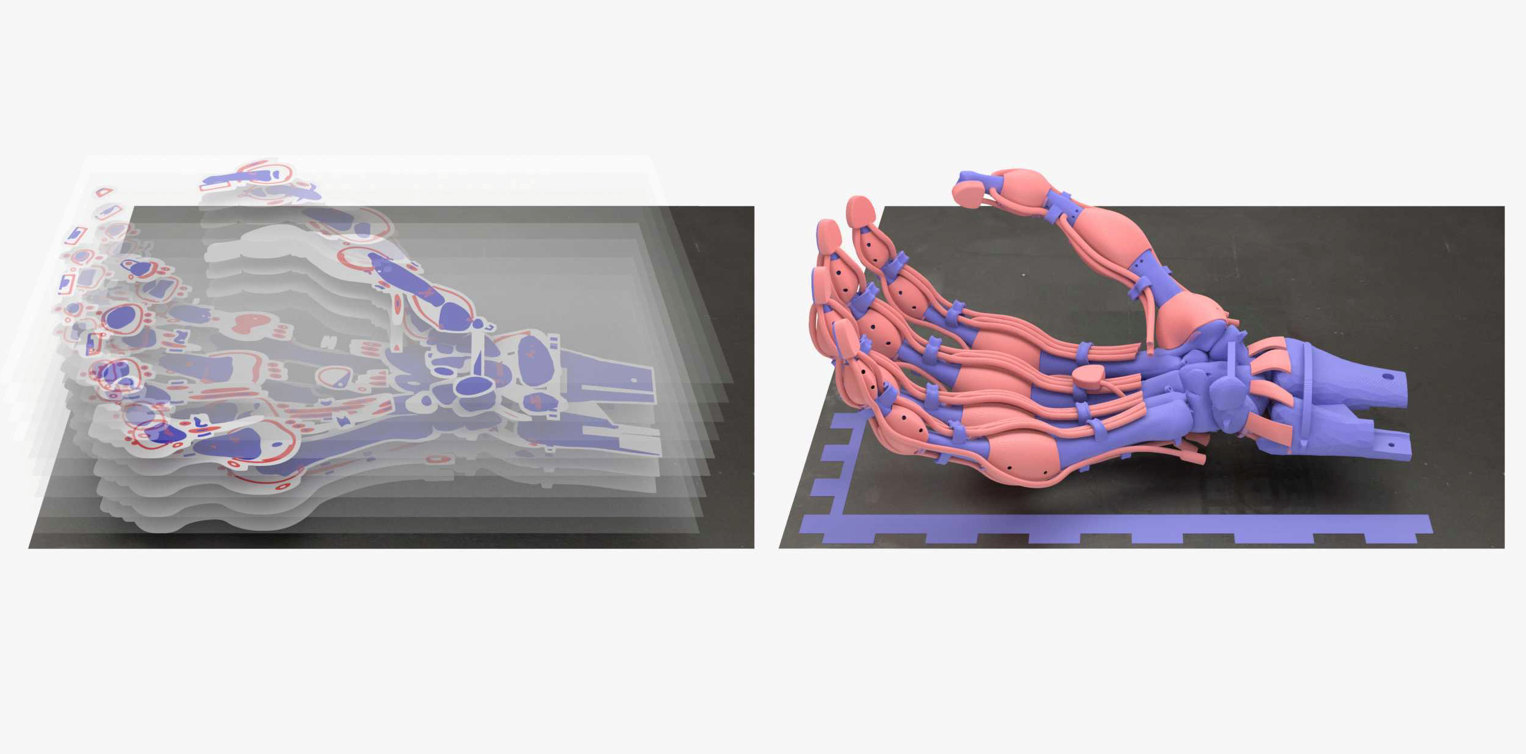
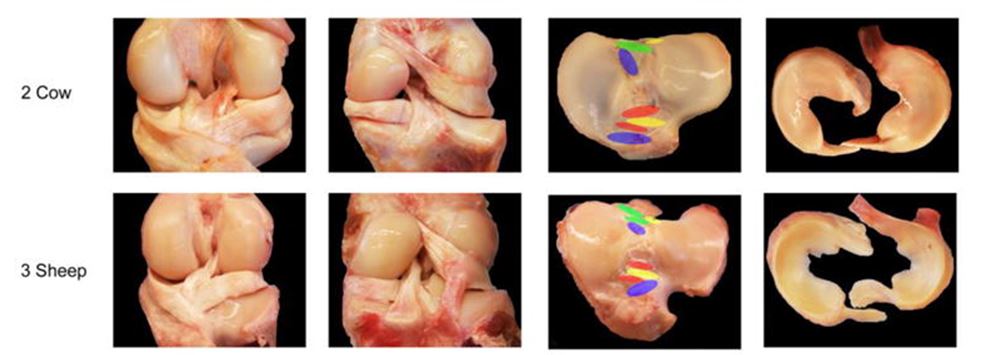
A notable application of this innovation is demonstrated by ETH Zurich researchers who have successfully 3D printed a robotic hand with integrated bones, ligaments, and tendons made from different polymers in a single operation. This achievement was made possible by utilizing slow-curing thiolene polymers, known for their excellent elastic properties and rapid return to their original state after bending.
Thomas Buchner, a doctoral student in the group of ETH Zurich robotics professor Robert Katzschmann, highlights the significance of these polymers: “We’re now using slow-curing thiolene polymers. These have very good elastic properties and return to their original state much faster after bending than polyacrylates.” This makes thiolene polymers ideal for producing the elastic ligaments of the robotic hand.
The flexibility of thiolenes in terms of stiffness allows for precise tuning, meeting the specific requirements of soft robots. Katzschmann explains the advantages of soft robots, emphasizing their reduced risk of injury when interacting with humans and suitability for handling fragile goods, making them superior to conventional metal robots.
Traditional 3D printing methods involved scraping off surface irregularities after each curing step, a process compatible only with fast-curing polyacrylates. To accommodate slow-curing polymers like thiolenes and epoxies, the researchers incorporated a 3D laser scanner into the printing process. This scanner immediately checks each printed layer for surface irregularities, enabling a feedback mechanism to make real-time adjustments to the amount of material printed in subsequent layers, without the need for smoothing uneven layers.
The new printing technology was developed by MIT spin-off, Inkbit, in collaboration with ETH Zurich researchers who optimized the technology for use with slow-curing polymers. Their joint efforts have been published in the journal Nature. Moving forward, Katzschmann’s group at ETH Zurich plans to explore further possibilities using this technology, developing more sophisticated structures and additional applications. Meanwhile, Inkbit intends to offer a 3D printing service to customers and market the new printers incorporating this advanced technology.
References:
- Buchner TJK, Rogler S, Weirich S, Armati Y, Cangan BG, Ramos J, Twiddy S, Marini D, Weber A, Chen D, Ellson G, Jacob J, Zengerle W, Katalichenko D, Keny C, Matusik W, Katzschmann RK: Vision-Controlled Jetting for Composite Systems and Robots, Nature, 15. November 2023, doi: external page10.1038/s41586-023-06684-3call_m

This article was written by Elisa Bissacco as part of an ongoing series of scientific communications written and curated by BioTrib’s Early Stage Researchers.
She is studying a PhD in Tribological Characteristics of Nanofibrous Electrospun Materials at ETH Zurich.