When engaging in discussions about my research, particularly with individuals outside of academia, often within my community, I frequently encounter a common question: “What is your involvement with artificial hip joints? Are you a doctor?” In response, I find myself explaining the various phases of developing load-bearing implants and clarifying the role I play as a mechanical research engineer. In this article, I aim to elucidate the developmental stages of implants, spanning from the laboratory to clinical application, and emphasize the valuable contributions made by engineers like myself.
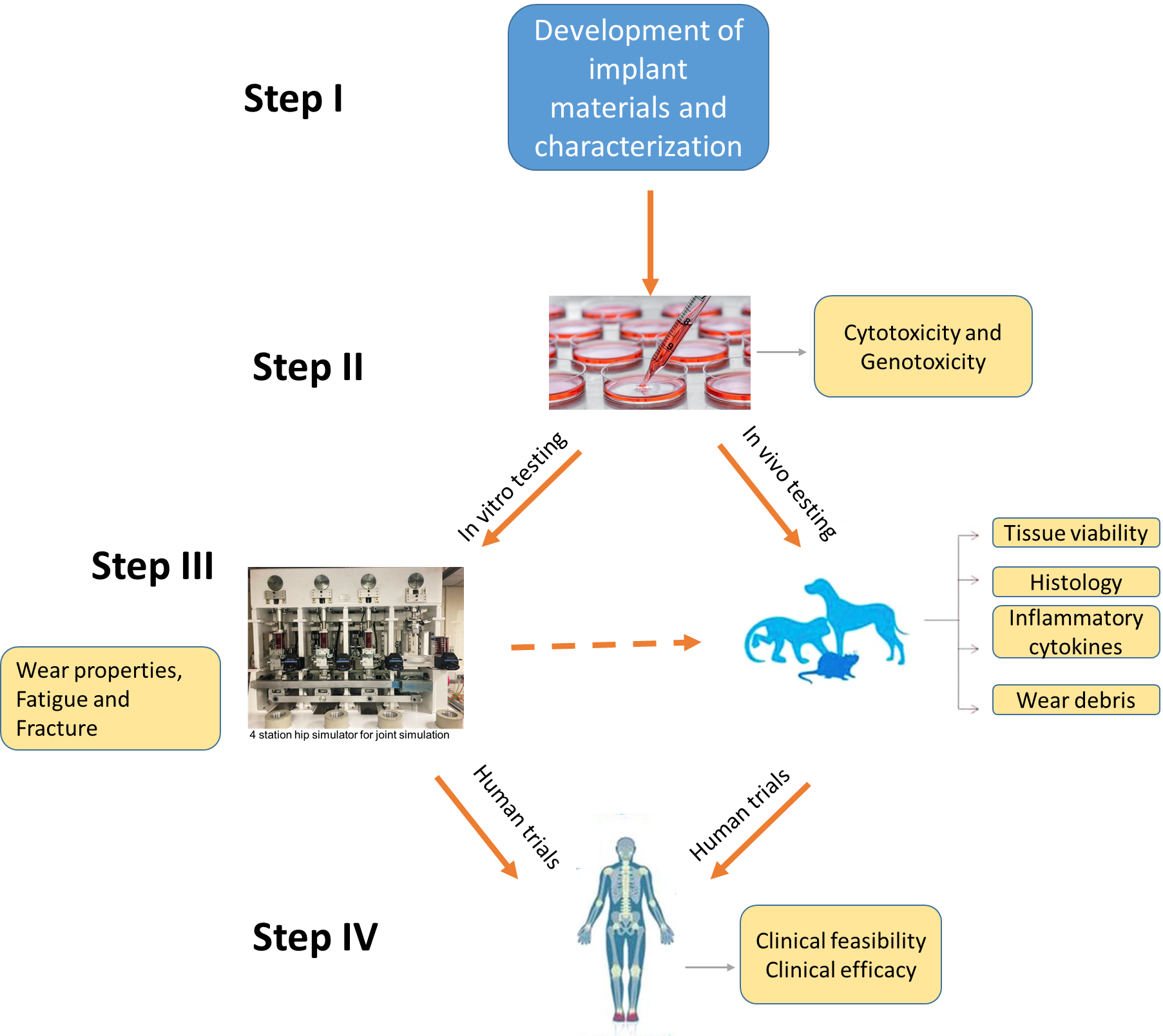
Biomedical implants undergo several essential stages before being suitable for implantation in the human body. These stages encompass material development and characterization, biocompatibility testing, biomechanical testing for load-bearing implants, in vivo implantation, and ultimately human trials. Throughout the initial three stages, engineers play a significant role, with a particular emphasis on biomechanical testing (Stage I-III in the figure). This is because before biomedical devices make their way into clinics and hospitals, rigorous testing is essential to ensure their safety, efficacy, and compatibility with real-world conditions. This is where biomechanical testing comes into play, serving as a bridge between laboratory experiments and real-world applications.
Biomechanical testing is a multidisciplinary approach that evaluates the mechanical behaviour and performance of medical devices in simulated physiological environments. It involves a combination of engineering principles, biology, and clinical knowledge to mimic the conditions the device will face within the human body. This testing is crucial to identify potential issues, assess the device’s functionality, and refine its design before it reaches the patient termed as preclinical testing. Such as Implants with moving parts (hip or knee prostheses) undergo wear testing to assess the materials’ performance and any potential debris generated by friction. This is crucial to avoid complications like inflammation or tissue damage. The tests are guided by several international standards. ISO 14242 primarily focuses on wear testing in a laboratory setting, it emphasizes the importance of ensuring that wear testing conditions are relevant to the clinical conditions experienced by patients with hip implants. Whereas ISO 7206 provides guidelines for the development, testing, and performance evaluation of hip joint implants to ensure their safety and efficacy.
Biomechanical testing is carried out using a range of mechanical simulators, including pin-on-plate tribometers, hip and knee simulators, and more. As researchers, we are deeply involved in these efforts. Furthermore, our team at the University of Leeds actively collaborates with patients and surgeons at Leeds Teaching Hospital, effectively bridging the gap between academia and the medical sector.
For further insights into the phases of implant development, I recommend reviewing the following article.
Hamadouche, Moussa, et al. “Alumina-on-alumina articulation in total hip arthroplasty: From bench-side to bedside.” Seminars in Arthroplasty. Vol. 17. No. 3-4. WB Saunders, 2006. https://doi.org/10.1053/j.sart.2006.09.006

This article was written by MM Raihan as part of an ongoing series of scientific communications written and curated by BioTrib’s Early Stage Researchers.
Raihan is researching In-situ Measurement of Nano-scale Wear Utilising Advanced Sensors at the University of Leeds, UK.