Biodegradable implants for bone fracture fixation are a relatively new technology that has the potential to revolutionize the way we treat bone fractures. These implants are made from biodegradable materials that are designed to dissolve over time, eliminating the need for additional surgery to remove them.
One of the main benefits of biodegradable implants is that they can help reduce the risk of infection and complications associated with traditional metal implants. They also avoid the need for a second surgery to remove the implant, which can be costly and time-consuming.
Another advantage of biodegradable implants is that they can provide support for the bone as it heals, while also promoting the growth of new bone tissue. This can help to improve the overall healing process and reduce the risk of complications such as non-union or malunion of the bone.
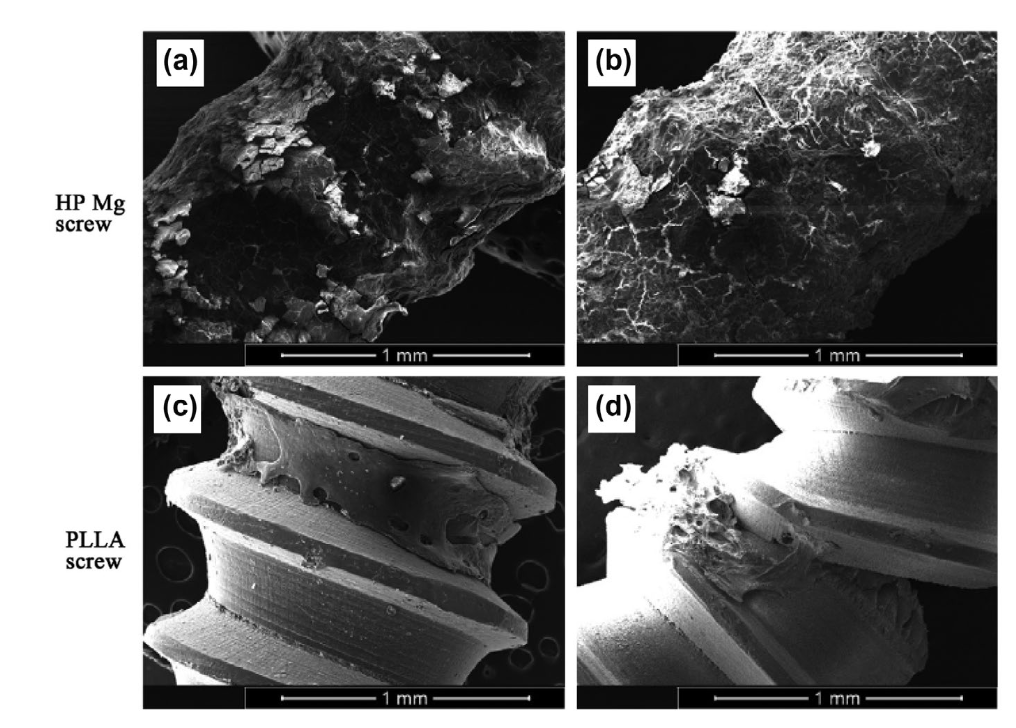
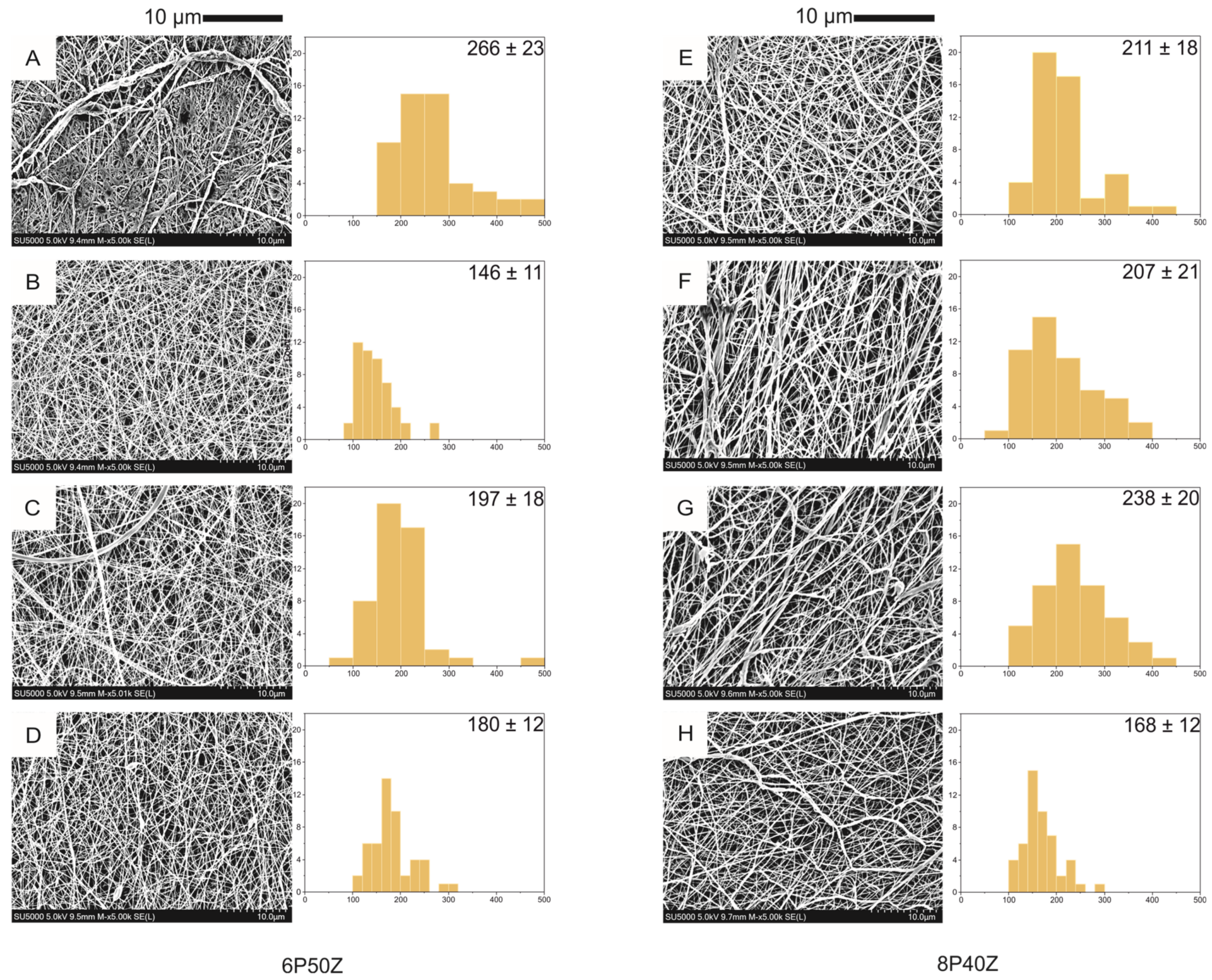
There are currently several types of biodegradable implants available for use in bone fracture fixation, including those made from polylactic acid (PLA), polycaprolactone (PCL), polydioxanone (PDO) and also metal based alternatives. Each of these materials has its own unique properties that make it suitable for different types of fractures and patients. Among the metal ones a lot of interest is raising around magnesium alloys that have the advantage of having appropriate mechanical properties, high biocompatibility and to promote bone in-growth.
It is important to note that biodegradable implants are not suitable for all types of fractures or patients. They are typically used for fractures that are considered to be low-risk and are not expected to experience high levels of stress during the healing process.
Despite these limitations, biodegradable implants are a promising technology that could have a significant impact on the way we treat bone fractures in the future. As research in this area continues to advance, we can expect to see even more innovative and effective biodegradable implant options for patients.
In conclusion, biodegradable implants for bone fracture fixation are a relatively new technology that holds great promise for the future. These implants are made from biodegradable materials that are designed to dissolve over time, eliminating the need for additional surgery to remove them. They can help reduce the risk of infection and complications associated with traditional metal implants, while promoting the growth of new bone tissue. With more research, the limitations of these implants can be overcome and they can be used to treat a wider range of fractures.
References:
[1] K. Kumar, R. S. Gill, and U. Batra, “Challenges and opportunities for biodegradable magnesium alloy implants,” Mater. Technol., vol. 33, no. 2, pp. 153–172, Jan. 2018, doi: 10.1080/10667857.2017.1377973.

This article was written by Giulio Cavaliere as part of a series articles curated by BioTrib’s Early Stage Researchers.
Giulio Cavaliere is investigating Additively manufactured biodegradable alloys for bone replacement at Uppsala University, Sweden.