PRG4 or lubricin is a protein with a bottle-brush shape that can be perfectly mimicked by polymer brush grafting to biomaterial surfaces. This imparts to biomaterials’ surfaces super lubricous properties and a coefficient of friction (µ) lower than 0.01. In addition, polymer brushes grafted to material surfaces may impart tunable hydrophilicity, self-cleaning, catalysis, controlled cell, and bacteria adhesion [1]. They can be applied for response actuation and drug delivery. Lubrication polymer brushes can be charged (positive and negative charges), amphiphilic, or act via steric hindrances[1]. The end properties can be controlled by molecular weight, grafting density, and radius of gyration.
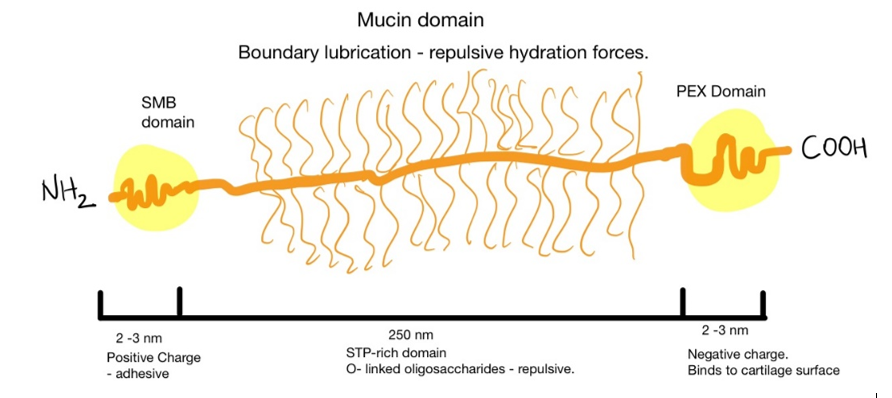
One of the mechanisms of lubrication is brush hydration. The thick water film would prevent the probe from contacting the surface[1]. In nature, this role is played by hyaluronic acid and other sugar molecules in articular cartilage, for example. The sugar molecules are conjugated to lubricin forming a mucinous domain. The protein is anchored by a somatomedin-B (SMB) domain to hyaluronic acid from the extracellular matrix of cartilage cells (chondrocytes) [2,3]. The glycosylated domains can trap water and establish electrostatic repulsion promoting lubricity of the tissue [4,5]
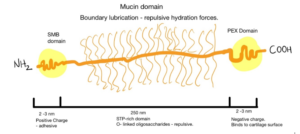
Several strategies have been developed to mimic this biochemical environment. Poly(l-lysine) (PLL) brushes were grafted onto poly(ethylene glycol) (PEG) surfaces to obtain good lubricity and biocompatibility[6]. Morgese et al. grafted to poly(glutamic acid) different polyoxazolines. These polymers are known for passivating surfaces and promoting now-fouling without eliciting immune responses. In samples with hydroxybutyrate (HBA), she obtained a biomimetic material that could bind to degraded cartilage and regenerate tissue[7]. This implies interesting solutions for people with early-onset arthritis.
To conclude, bottle-brush materials might be the future of cartilage tissue engineering, but more studies need to be conducted to show the in vivo feasibility of the concepts. We expect these new materials to influence scaffolds/gels potentially entering the market in the next decades.
REFERENCES
[1] S. Ma, X. Zhang, B. Yu, F. Zhou, Brushing up functional materials, NPG Asia Mater. 11 (2019) 24. https://doi.org/10.1038/s41427-019-0121-2.
[2] Y. Lee, J. Choi, N.S. Hwang, Regulation of lubricin for functional cartilage tissue regeneration: a review, Biomater Res. 22 (2018) 9. https://doi.org/10.1186/s40824-018-0118-x.
[3] I. Bayer, Advances in Tribology of Lubricin and Lubricin-Like Synthetic Polymer Nanostructures, Lubricants. 6 (2018) 30. https://doi.org/10.3390/lubricants6020030.
[4] S.M.T. Chan, C.P. Neu, G. DuRaine, K. Komvopoulos, A.H. Reddi, Atomic force microscope investigation of the boundary-lubricant layer in articular cartilage, Osteoarthritis and Cartilage. 18 (2010) 956–963. https://doi.org/10.1016/j.joca.2010.03.012.
[5] I.M. Schwarz, B.A. Hills, Surface-active phospholipid as the lubricating component of lubricin, Rheumatology. 37 (1998) 21–26. https://doi.org/10.1093/rheumatology/37.1.21.
[6] S. Lee, M. Müller, M. Ratoi-Salagean, J. Vörös, S. Pasche, S.M. De Paul, H.A. Spikes, M. Textor, N.D. Spencer, Boundary Lubrication of Oxide Surfaces by Poly(L-lysine)-g-poly(ethylene glycol) (PLL-g-PEG) in Aqueous Media, Tribology Letters. 15 (2003) 231–239. https://doi.org/10.1023/A:1024861119372.
[7] G. Morgese, E. Cavalli, J.-G. Rosenboom, M. Zenobi-Wong, E.M. Benetti, Cyclic Polymer Grafts That Lubricate and Protect Damaged Cartilage, Angew. Chem. 130 (2018) 1637–1642. https://doi.org/10.1002/ange.201712534.

This article was written by André Plath as part of an ongoing series of scientific communications written and curated by BioTrib’s Early Stage Researchers.
André is researching Boundary Lubrication of Fibrous Scaffolds at ETH Zürich, Switzerland.


 This article was written by Rob Elkington, the BioTrib website manager as part of a series of blog posts for LGBTQ+ history month.
This article was written by Rob Elkington, the BioTrib website manager as part of a series of blog posts for LGBTQ+ history month.
 This post was written by Esperanza Shi as part of an ongoing series of scientific communications written and curated by BioTrib’s
This post was written by Esperanza Shi as part of an ongoing series of scientific communications written and curated by BioTrib’s 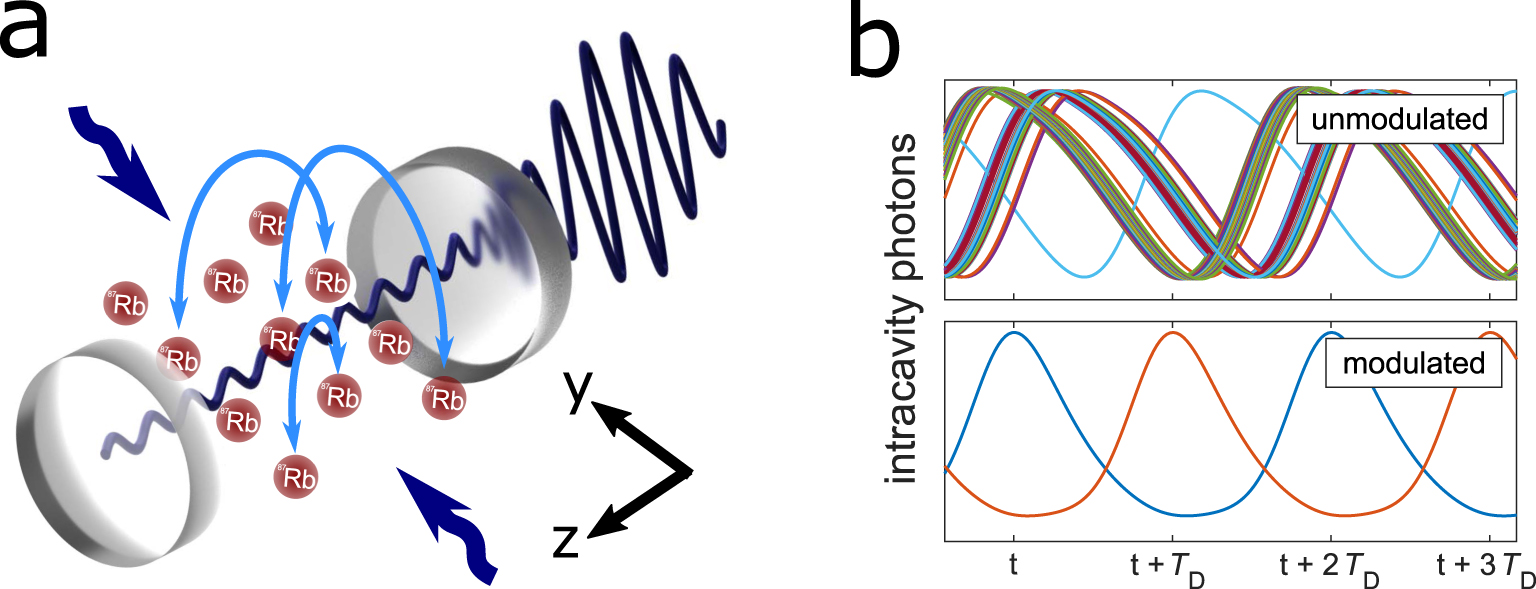
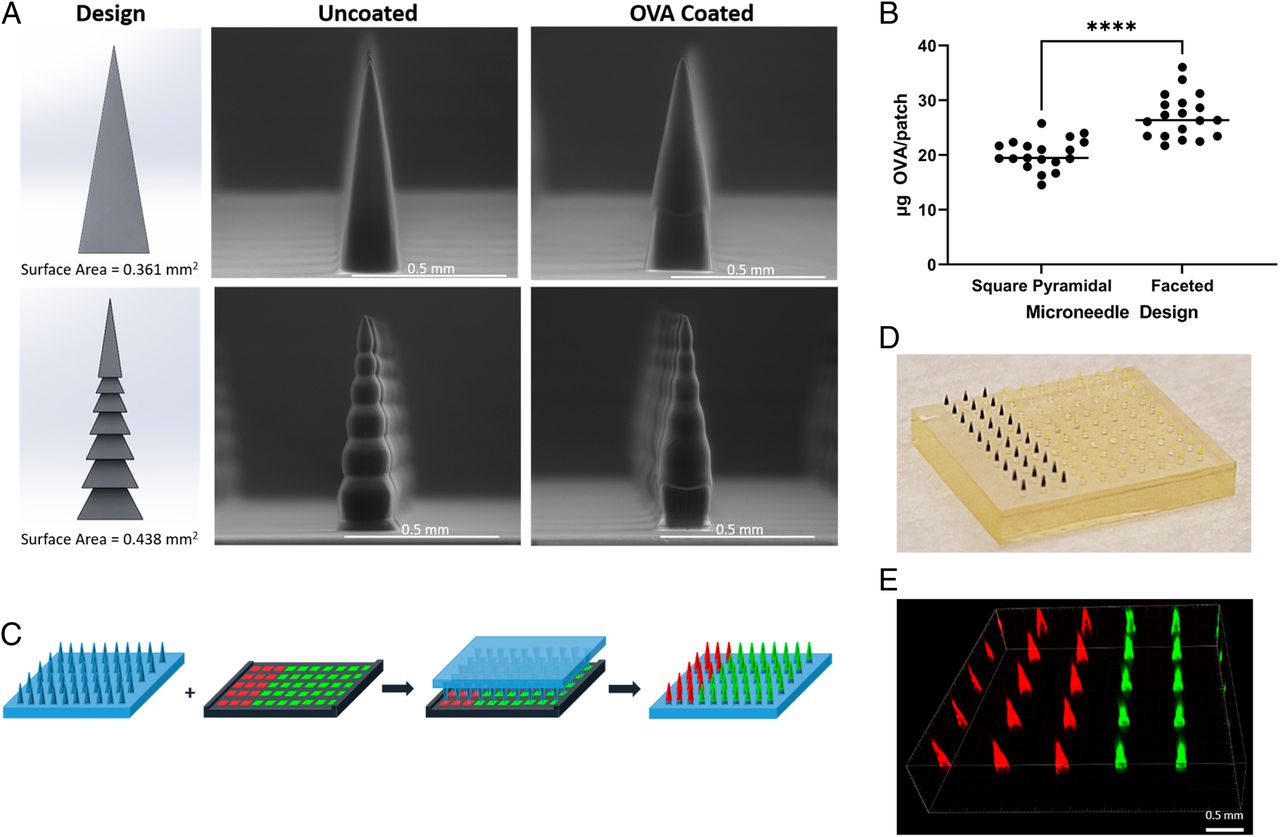
 This post was written by Pedro Luiz Lima dos Santos as part of an ongoing series of scientific communications written and curated by BioTrib’s
This post was written by Pedro Luiz Lima dos Santos as part of an ongoing series of scientific communications written and curated by BioTrib’s