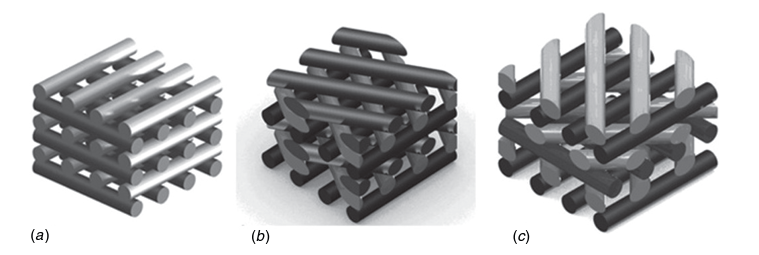
Header Image: Schematic representation of scaffolds characterized by three different lay-down patterns. (a) 0◦/90◦. (b) 0◦/60◦/120◦. (c) 0◦/45◦/90◦/135◦. [1]
The main objective of 3D bioprinting is to recreate human tissues with the same mechanical, structural and biological properties as the corresponding native tissue, in order to solve the problems associated with conventional transplantation techniques (donor site morbidity, organ shortage, etc.). For this purpose, different cell types combined with different biomaterials have been bioprinted according to specific models, but obtaining a 3D scaffold with the desired properties remains a challenge.
The advantage of 3D bioprinting over conventional scaffold fabrication techniques is the ability to control the 3D architecture of scaffolds through parameters such as pore size and geometry. Pore size and shape influence the resulting mechanical properties as well as cell behavior and tissue growth over time. Domingos et al. showed that for a lay down pattern of 0◦/90◦ (filament orientation between layers) with different pore sizes, poly(ε-caprolactone) scaffolds with smaller pores exhibit significantly higher stiffness under compressive conditions, which is an important property in applications such as bone tissue engineering. For different pore shapes with the following lay down pattern: 0◦/90◦, 0◦/60◦/120◦ and 0◦/45◦/90◦/135◦ (see figure) and the same porosity, the increasing number of angles between the filaments of the different layers leads to an increase in the deformability of the construct under compressive conditions.
Regarding the influence of architecture on cell behavior, viability and proliferation of human mesenchymal stem cells (hMSCs) were studied for 21 days and showed that larger pores with a lay down pattern of 0◦/90◦ improve viability and proliferation.
References
[1] Domingos, M., et al. “THE FIRST SYSTEMATIC ANALYSIS OF 3D RAPID PROTOTYPED POLY (e-CAPROLACTONE) SCAFFOLDS MANUFACTURED THROUGH BIOCELL PRINTING: EFFECT OF PORE SIZE AND GEOMETRY ON COMPRESSIVE MECHANICAL BEHAVIOR AND IN VITRO HMSC VIABILITY.” 1758-5082 5 (2013).

This article was written by Marie Moulin as part of an ongoing series of scientific communications written and curated by BioTrib’s Early Stage Researchers.
Marie is researching the Bioprinting of Bone and Cartilage at Uppsala University, Sweden.