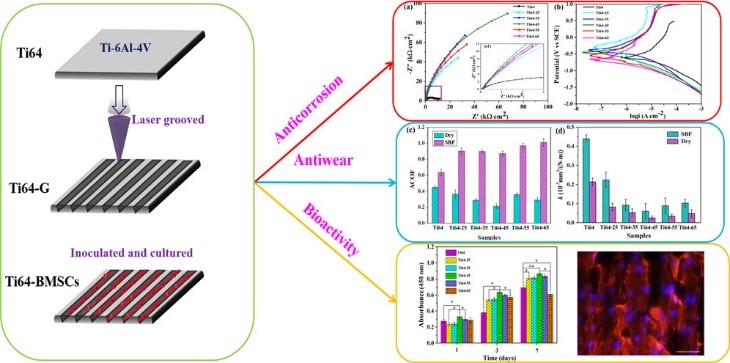
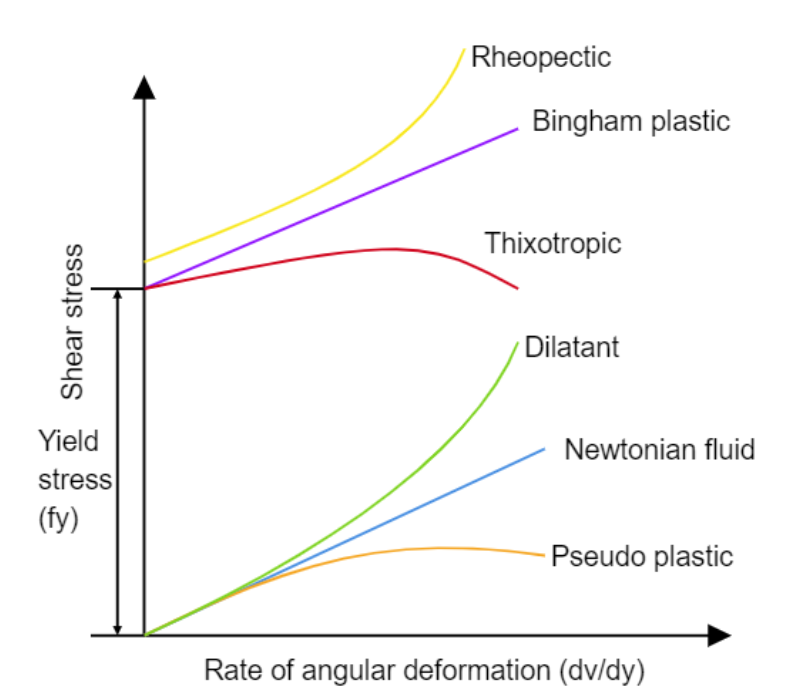
Articular cartilage is a living material composed of a relatively small number of cells known as chondrocytes surrounded by a multicomponent matrix. Mechanically, articular cartilage is a composite of materials with widely differing properties. Approximately 70 to 85% of the weight of the whole tissue is water. The remainder of the tissue is composed primarily of proteoglycans and collagen. Proteoglycans consist of a protein core to which glycosaminoglycans (chondroitin sulfate and keratan sulfate) are attached to form a bottlebrush-like structure.
The structure of articular cartilage is often described in terms of four zones between the articular surface and the subchondral bone: the surface or superficial tangential zone, the intermediate or middle zone, the deep or radiate zone, and the calcified zone.
Currently, the most used techniques for articular cartilage regeneration are microfracture (MF), osteochondral autologous transplantation (OAT), osteochondral allograft transplantation (OCA), particulate articular cartilage implantation (PACI), and autologous chondrocyte implantation (ACI). However, these methods have limitations including calcification, formation of transient fibrocartilaginous tissue, and the low capacity of binding to surrounding normal cartilage [1, 2]. Therefore, scaffolds with improved bulk mechanical properties could increase the efficacy of treatment and promote an earlier return to normal activity.
In recent years, tissue engineering technology has been considered the most promising method for regenerating the articular cartilage [3] [4].
In tissue engineering applications, biomaterial scaffolds play animportant role in providing a 3D environment that supports cellgrowth, matrix deposition, and tissue regeneration. An ideal tissue engineering scaffold should meet several important criteria:
- Be biocompatible, minimizing local tissue reactions andmaximizing cell growth and tissue integration.
- Be biodegradable with good absorption rate, providing support for early cell proliferation and allows for gradualdegradation after the formation of new tissue.
- Have adequate porosity and interconnectivity to allow cellmigration and efficient exchange of nutrients and waste.
- Possess suitable mechanical properties to support tissue growthunder natural mechanical loads.
To date, many biomaterial scaffolds have been extensively studied, including natural polymers extracted from living organisms and synthetic materials derived from various chemical processes used intissue repair and regeneration.
Natural biomaterials are popular as scaffolds for cartilage repair and regeneration due to their excellent biocompatibility for cell adhesion and differentiation. In particular, natural scaffolds used in tissue engineering of articular cartilage include carbohydrate-based hyaluronic acid, agarose, alginate, chitosan, and protein-based collagen or fibrin glues.
Due to its ease of fabrication and chemical modification, excellent biocompatibility, high versatility, suitable mechanical properties and controllable biodegradability, synthetic polymers are currently being investigated for their potential as a scaffold for cartilage tissue. The most common synthetic polymers for cartilage engineering scaffolds are polylactic acid (PLA, present in both L and D forms), polyglycolicacid (PGA), and its copolymer poly-lactic-co- Glycolic acid (PLGA).
Conventional natural or synthetic scaffolds still need to be improved to achieve better biocompatibility and functional properties for cartilage regeneration. Because the size of native cartilage tissue is only nanometers, and chondrocytes directly interact with nanostructured ECM, the biomimetic properties and excellent physicochemical properties of nanomaterials are essential for chondrocyte growth. [5].
Solution electrospinning (SES) is a technique that allows the production of nanofibrous scaffolds and allows for the tuning of the 3D scaffolds by changing the fiber diameter and scaffold porosity. The process consists of a pump that pushes out, through a metal needle (spinneret), the polymer solution, inserted in a syringe. The presence of a high voltage source that energizes the polymer solution causes formation of a conical jet (Taylor cone) which is then drawn into a fiber by electrostatic repulsion [5] [6]. The resulting fibers are deposited on a flat or tubular electrode (collector). The thin electrospun fibers range from a few hundred nanometers to a few micrometers and are suitable candidates to mimic the structure of the natural extracellular matrix (ECM) as they can stimulate cell ingrowth and proliferation [7].
Advances in fabrication methods have solved the scalability problem and enabled the development of porous structures that allow long-term cell invasion and growth. Despite all these advantages, electrospun scaffolds have yet to be fully evaluated in preclinical models and clinical settings, hindering widespread acceptance of this breakthrough technology in biomedicine [8]
Bibliography
[1] C. Vinatier and J. Guicheux, “Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments,” Annals of Physical and Rehabilitation Medicine, vol. 59, pp. 139 – 144, 2016.
[2] W. Wei, Y. Maa, X. Yao, W. Zhou, X. Wang, L. Chenglin, J. Lin, Q. He, S. Leptihna and H. Ouyang, “Advanced hydrogels for the repair of cartilage defects and regeneration,” Bioactive Materials, vol. 6, p. 998–1011, 2013.
[3] S. Jiang, W. Guo, G. Tian, X. Luo, L. Peng, S. Liu, X. Sui, Q. Guo and X. Li, “Clinical Application Status of Articular Cartilage Regeneration Techniques: Tissue-Engineered Cartilage Brings New Hope,” Stem Cells International, 2020.
[4] A. Martín, H. Zlotnick, J. Carey and R. Mauck, “Merging therapies for cartilage regeneration in currently excluded ‘red knee’ populations,” Nature Partner Journal Regenerative Medicine, vol. 4, 2019.
[5] N. Maurmann, S. L and P. P, “Electrospun and Electrosprayed Scaffolds for Tissue Engineering,” Cutting-Edge Enabling Technologies for Regenerative Medicine, pp. 79 – 100, 2018.
[6] R. Soares and al., “Electrospinning and electrospray of bio-based and natural polymers for biomaterials development,” Mater Sci Eng C Mater Biol Appl, pp. 969-982, 2018.
[7] D. Alexeev and al., “Electrospun biodegradable poly(epsilon-caprolactone) membranes for annulus fibrosus repair: Long-term material stability and mechanical competence,” JOR Spine, vol. 1, 2021.
[8] E. Z. D. Yilmaz, “Electrospun Polymers in Cartilage Engineering—State of Play,” Front. Bioeng. Biotechnol., 2020.
[9] L. H. J. A. K. Zhang, “The Role of Tissue Engineering in Articular Cartilage Repair and Regeneration,” NIH Public Access, 2009.

This article was written by Elisa Bissacco as part of an ongoing series of scientific communications written and curated by BioTrib’s Early Stage Researchers.
She is studying a PhD in Tribological Characteristics of Nanofibrous Electrospun Materials at ETH Zurich.
 This post was written by Pedro Luiz Lima dos Santos as part of an ongoing series of scientific communications written and curated by BioTrib’s Early Stage Researchers.
This post was written by Pedro Luiz Lima dos Santos as part of an ongoing series of scientific communications written and curated by BioTrib’s Early Stage Researchers.